Work done in a constant volume process is
a. negative
b. zero
c. positive
d. none of the above
Correct Answer : b. zero
Explanation :When a thermodynamic system undergoes a path from one state to another state work is done either by the system or on the system. When we plot this path on P-V diagram then the area under this curve is the work involved in transformation. In constant volume process the path of change of state will be,
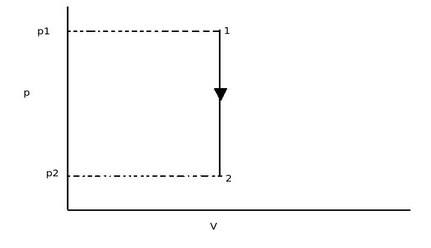
From above diagram, as the constant volume process, the initial volume V1 is equal to final volume V2. Thus there is no area under the curve. Thus work done in constant volume process is zero.