1) Work done in a constant volume process is
a. negative
b. zero
c. positive
d. none of the above
|
Answer
Explanation
|
ANSWER: zero
Explanation:
When a thermodynamic system undergoes a path from one state to another state work is done either by the system or on the system. When we plot this path on P-V diagram then the area under this curve is the work involved in transformation. In constant volume process the path of change of state will be,
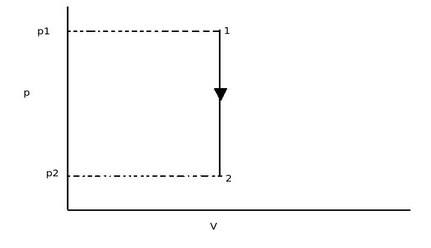
From above diagram, as the constant volume process, the initial volume V1 is equal to final volume V2. Thus there is no area under the curve. Thus work done in constant volume process is zero.
|
|
2) The amount of heat transferred to convert unit mass of solid to vapour or vice versa is called as
a. latent heat of vaporization
b. latent heat of fusion
c. latent heat of sublimation
d. specific heat
|
Answer
Explanation
|
ANSWER: latent heat of sublimation
Explanation:
The amount of heat transfer required to cause a phase change in unit mass of a substance at constant volume and temperature is called as latent heat. There are three types of latent heat as there are three phases solid, liquid, gas or vapour. The amount of heat transfer required to melt unit mass into liquid or liquid into solid is the latent heat of fusion (lfu). The latent heat of vaporization (lvap) is the amount of heat transfer required to vaporise unit mass of liquid into vapour or vice versa. The latent heat of sublimation (lsub) is the amount of heat transfer required to convert unit mass of solid into vapour of vice versa.
|
|
3) The amount of heat required to raise a unit mass of substance through a unit rise in temperature is called as
a. heat capacity of a substance
b. specific heat of a substance
c. latent heat of a substance
d. none of the above
|
Answer
Explanation
|
ANSWER: specific heat of a substance
Explanation:
Specific heat of the substance is defined as the amount of heat required to raise a unit mass of substance through a unit rise in temperature. The symbol used for specific heat is 'c'.
c = Q / (m . Δt) J/kg K
Where Q is the amount of heat in J, m is the mass of substance in kg, t is the temperature in K.
For gases, if the process is at constant pressure then specific heat is
cp. If the process is at constant volume, then it is cv.
The product of mass (m) of the substance and specific heat (c) is heat capacity. The capital letters, C , Cp , Cp are used for heat capacity.
|
|
4) The equation for calculating amount of heat transfer through a system boundary
when,
T is temperature, an intensive property
X is an extensive property which is result of heat transfer is
a. Q1-2 = 1∫2 T dX
b. Q1-2 = 1∫2 X dT
c. Q1-2 = 1∫2 (1/T) dX
d. none of the above
|
Answer
Explanation
|
ANSWER: Q1-2 = 1∫2 T dX
Explanation:
Heat transfer is also a path function like work transfer. The work involved in a thermodynamic process is give by the equation,
W1-2 = 1∫2 P dV
where,
p is the pressure and V is the volume of the system.
Whenever there is change in pressure, there will be displacement work. Therefore, pressure difference is a cause and the work transfer is the effect and the change in volume is a result. The quantity of the heat transfer can also be determined as work transfer. In heat transfer process, whenever there is temperature difference, heat transfer takes place and a change is certain extensive property takes place. Thus, temperature is the cause and heat transfer is the effect. Therefore, the heat flow can be determined by Q1-2 = 1∫2 T dX.
|
|
5) Heat transfer is
a. a point function
b. a path function
c. a transfer function
d. none of the above
|
Answer
Explanation
|
ANSWER: a path function
Explanation:
The quantity of heat transfer that is heat flow Q can be determined in terms of the work W in the same process. Work involved in a process is not the same because it depends upon the path through which the process is carried out. The work involved in a process is not conserved. But, the difference (Q – W) is conserved for all the paths between the two states in a process, because of principle of conservation of energy. Therefore, heat flow Q, like W, depends on the path through which the process is carried out. Heat flow is path-dependent not a property. Therefore heat transfer is a path function. The amount of heat transfer when a system changes from state 1 to state 2 depends on the paths through which the system passes.
|
|
6) The process in which no heat transfer takes place through boundaries is called as
a. isothermal process
b. adiabatic process
c. isochoric process
d. none of the above
|
Answer
Explanation
|
ANSWER: adiabatic process
Explanation:
In adiabatic process, no heat crosses the boundaries of the system. Therefore no heat transfer takes place in adiabatic process. There is only work interaction between system and surrounding in adiabatic process. And the wall or boundary which does not allow the heat to flow through it is called as adiabatic wall and the wall which allows the heat to flow through it is called as diathermic wall. Isothermal process is the process in which temperature is constant. In isothermal process, the heat flows into or out of the system is very slow to maintain thermal equilibrium. Isochoric is the constant volume process.
|
|
7) Heat flow is a quantity of heat transfer
a. within definite time
b. within definite cross-sectional area
c. within definite volume of the system
d. none of the above
|
Answer
Explanation
|
ANSWER: within definite time
Explanation:
The direction of the heat transfer takes place from a body with higher temperature to a body with lower temperature. The heat transfer takes place through all directions of the system with different rates of heat transfer. Heat transfer with a certain rate is a heat flow. Heat flow can be defined as a quantity of heat transfer within definite time. Q is the symbol of heat flow. If heat flows into a system then it is considered positive and if heat flows out of the system then it is considered as negative.
|
|
8) Heat is transferred across a boundary by virtue of a temperature difference. The heat is transferred, that means
a. force transfer takes place
b. energy transfer takes place
c. temperature transfer takes place
d. all of the above
|
Answer
Explanation
|
ANSWER: energy transfer takes place
Explanation:
When a system changes its state from state 1 to state 2, the work involved depends upon the path through which the system undergoes change. For consistency with the principle of conservation of energy, some other type of energy transfer along with work transfer must have taken place between the system and surrounding. This type of 'energy transfer' is occurred by virtue of a temperature difference. This energy is called as 'heat'.
|
|
9) In free expansion of gas, the work transfer is
a. negative
b. zero
c. positive
d. none of the above
|
Answer
Explanation
|
ANSWER: zero
Explanation:
When a gas is expanded against vacuum then this expansion is called as free expansion. Work transfer is a boundary function as it is determined only at boundary. Let us consider a gas is separated from vacuum by a partition. Consider this as a system. When this partition is removed, the gas quickly fills up the vacuum area. If we do not consider the work related to removing the partition, then there is no work transfer involved here, because no work crosses the system boundary. Work is done by a system to overcome some resistance and to fill up the vacuum there is no any resistance to the gas to overcome. Therefore work transfer is zero in free expansion.
|
|
10) Which among the following systems undergo/es work transfer?
a. Current flowing through the resistor
b. A shaft rotated by a motor
c. both a. and b.
d. none of the above
|
Answer
Explanation
|
ANSWER: both a. and b.
Explanation:
When electric current passes through the resistor, work transfer can takes place. This is because the current can drive a motor and the motor can drive a pulley and this can results in lifting a weight.
When a shaft is rotated by a system, work transfer can takes place into the system. This is because the shaft can rotate a pulley which can lift a weight. Thus both the systems undergo work transfer.
|
|