Free expansion of gas within a system is
Options:a. a reversible process
b. an irreversible process
c. a quasi-static process
d. none of the above
Correct Answer: b. an irreversible process
 Explanation
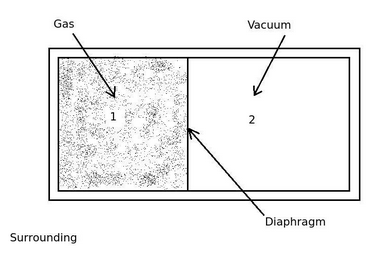
ExplanationConsider the above diagram, a system contains gas in the compartment 1 and vacuum is separated by a diaphragm in compartment 2. If the diaphragm is removed then the gas in compartment 1 will expand into the compartment 2 until the pressure in both the compartments will become equal. This is a spontaneous process.
It is not possible to return the gas in the compartment 2 into the compartment 1 with increase in the pressure, without any other effect on the system. To return this process, the external work has to be done on the system. Therefore it a irreversible process.