Property of entropy - MCQs with Answers
Q1. When a system is taken from state A to state B through a reversible path 1 and again the system is taken to its initial state A from B through different reversible path 2, then what will be the effect on entropy?a. entropy increases
b. entropy decreases
c. entropy remains constant
d. none of the above
View Answer / Hide Answer ANSWER: c. entropy remains constant
Q2. Two reversible paths P1 and P2 are represented in the figure and the process represented is a cyclic process. Which will be the correct relation for the cyclic process shown?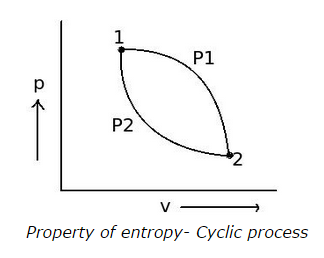
a. Cyclic ∫
P1 P2 (dQ/T) = 0
b.
1∫
2 P1 (dQ/T) =
1∫
2 P2 (dQ/T)
c.
1∫
2 P1 (dQ/T) =
2∫
1 P2 (dQ/T)
d. all of the above
View Answer / Hide Answer ANSWER: d. all of the above
Q3. Which type of function the entropy is?a. a path function
b. a point function
c. both a. and b.
d. none of the above
View Answer / Hide Answer ANSWER: b. a point function
Q4. 100 kg of ice at 0 °C is melted completely. What will be the change in entropy in kJ/K, when T2 = 0 °C and latent heat of fusion of ice is taken as 334.774 J/ga. 0
b. 100.6
c. 122.6
d. 150.6
View Answer / Hide Answer Q5. The entropy is a. an intensive property
b. an extensive property
c. both a. and b.
d. none of the above
View Answer / Hide Answer ANSWER: b. an extensive property
Q6. How is the entropy change of any irreversible process estimated, when the process is connecting two equilibrium points A and B?a. by replacing the original process by reversible zigzag path containing reversible adiabatic followed by isothermal and then again reversible adiabatic process between the same equilibrium points A and B
b. by replacing a reversible path between the same equilibrium points A and B
c. both a. and b.
d. none of the above
View Answer / Hide Answer ANSWER: b. by replacing a reversible path between the same equilibrium points A and B